APRIL 24, 2025
Pharma 4.0: How Smarter Maintenance Is Delivering Results
AKASH SHRIVASTAVA, PDM DATA SCIENTIST @ PETASENSE

The Pharma Industry’s Shift Toward PdM
In the pharmaceutical industry, predictive maintenance (PdM) plays a critical role in maintaining the reliability and efficiency of production processes. Unexpected equipment failures and downtime can lead to lost batches, putting the timely supply of life-saving medications at risk. This not only impacts the bottom line but also poses serious risks to regulatory compliance and public safety. Implementing PdM strategies through condition monitoring helps ensure equipment runs at peak performance, reducing the risk of unexpected breakdowns and keeping production schedules on track.
In response to the growing need for advanced maintenance strategies, the International Society for Pharmaceutical Engineering (ISPE) introduced the Pharma 4.0 initiative. The initiative encourages pharmaceutical companies to adopt Industrial Internet of Things (IIoT) technologies, placing PdM as a central component. PdM leverages machine data and advanced analytics to reduce downtime and improve overall equipment effectiveness. This shift toward IIoT-enabled PdM aligns with Pharma 4.0’s broader goals of improving efficiency, ensuring compliance, and maintaining high product quality.
This article explores the role of predictive maintenance (PdM) in the pharmaceutical industry, along with key challenges and proven solutions. It includes case studies where early fault detection and machine diagnostics helped leading pharmaceutical companies take timely action, avoiding production losses and costly equipment failures. These real-world examples show how condition monitoring and PdM help prevent operational disruptions and ensure continuous, high-quality production.
Why Predictive Maintenance Matters in Pharma
In the pharmaceutical industry, production relies on a wide range of highly specialized equipment that must operate reliably and within strict performance thresholds. Critical assets such as air handling units (AHUs), process pumps, compressors, agitators, fermenters and cooling tower fans are essential to maintaining environmental control and process integrity. Failures in these machines, whether due to hydraulic issues (such as unstable flow), structural resonance (from flexible or cantilevered mounting), or common mechanical defects like bearing wear and misalignment, can lead to batch loss, downtime, or regulatory non-compliance. Predictive maintenance helps mitigate these risks by continuously monitoring the health of critical machinery and detecting early signs of failure. This allows for timely maintenance actions that reduce unplanned downtime and ensure consistent product quality and compliance.

Compliance with strict regulatory standards is essential in pharmaceutical manufacturing. Predictive maintenance supports this by helping equipment stay within required operating parameters, reducing the risk of deviations that could result in regulatory violations. Consistent equipment performance is especially important in precision-driven processes like homogenization, where even slight variations can impact product quality. By proactively addressing equipment issues, PdM helps pharmaceutical manufacturers uphold the high standards of quality and reliability expected by regulatory bodies.
Still, implementing condition monitoring and PdM in the pharmaceutical sector presents several challenges. Handling large volumes of data requires a strong infrastructure for reliable transmission, processing, and storage. Monitoring assets that are hard to access or operate intermittently also adds complexity. There’s also a need for site-specific expertise and greater collaboration across facilities to standardize training, processes, and maintenance strategies. Overcoming these challenges is essential to fully realize the benefits of PdM and support the broader goals of Pharma 4.0.
Smarter Maintenance at Scale
Advanced IIoT-based predictive maintenance (PdM) technologies are becoming increasingly well-suited for the pharmaceutical industry. These modern systems combine wireless sensors with intelligent analytics to monitor key equipment parameters, such as vibration, temperature, and running speed, in real time. By enabling continuous condition monitoring of critical production and utility assets, these tools help detect early signs of equipment degradation, ensuring timely interventions and minimizing production disruptions.
Many PdM platforms support a centralized “hub” approach, allowing multiple pharmaceutical sites within an organization to be monitored and managed from a single platform by a specialized team of subject matter experts. This enables cross-site collaboration, promotes standardization, and enhances decision-making with real-time visibility into asset health across facilities.
In recent years, PdM technologies have evolved to address the specific needs of pharmaceutical manufacturers. Modern platforms now support GMP compliance, cleanroom compatibility, and actionable insights for a wide range of equipment. These technologies are deployed across two primary categories of assets:
1) Auxiliary equipment: supporting cleanroom environments and sterile utility delivery, such as HVAC fans, AHUs, and pumps used in WFI, RO, CIP, and chilled water systems.
2) Production and process equipment: including lyophilizers, agitators, fermenters, compressors, and other assets where uptime and precision directly impact product quality and yield.
Across both areas, Petasense has worked closely with leading pharmaceutical manufacturers to improve equipment reliability, reduce downtime, and support compliance with regulatory standards.
Purpose-Built for Pharma Operations
At Petasense, we’ve developed specialized features to meet the unique challenges of monitoring pharmaceutical equipment and ensuring measurement accuracy in complex environments. One such feature is state-based measurements, which allow sensors to capture data only when machines are running. This eliminates the noise caused by idle-state readings and enables more consistent comparisons over time. Integrated speed detection takes this further by triggering measurements only during specific operating speeds, ensuring apples-to-apples comparisons that reflect true machine behavior.
Another key capability is synchronized measurements. Multiple sensors installed on the same asset can coordinate via Bluetooth to capture data at the exact same moment. This ensures that vibration signals from motors, pumps, or gearboxes are collected under identical operating conditions, making the resulting diagnostics more reliable and actionable.
This is more than just smart features. It’s a proven system built for the realities of pharmaceutical manufacturing. In the next section, we’ll look at how pharmaceutical manufacturers are applying these technologies to detect faults early and eliminate costly downtime.
Case Study 1: Glycol Pump – Bearing Issue
At a pharmaceutical facility specializing in rare disease treatments, glycol pumps play a critical role in water circulation (to prevent freezing), gas dehydration, and solvent handling across various operations.
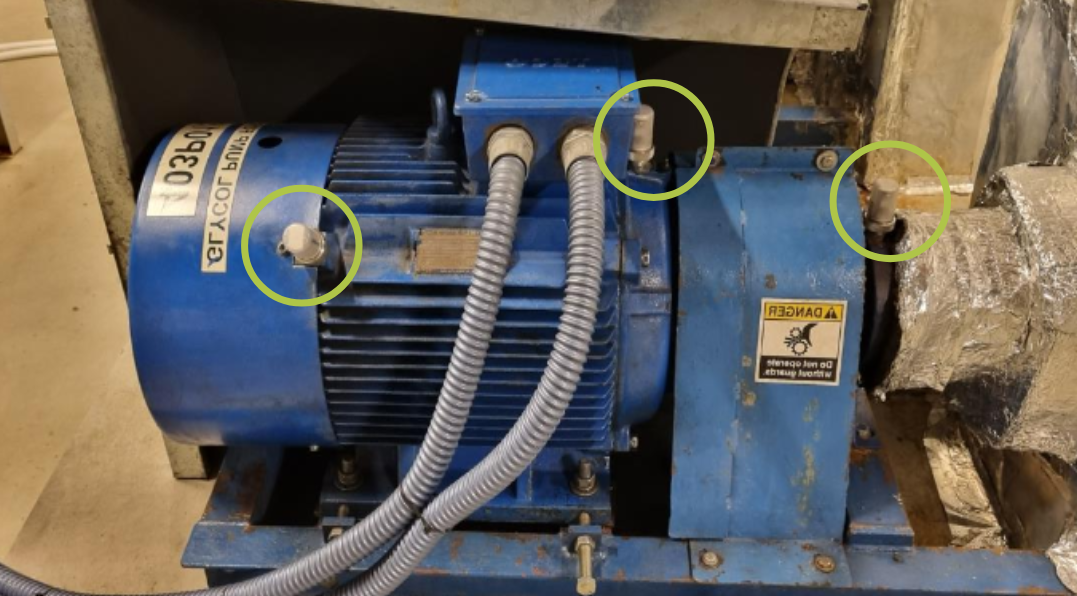
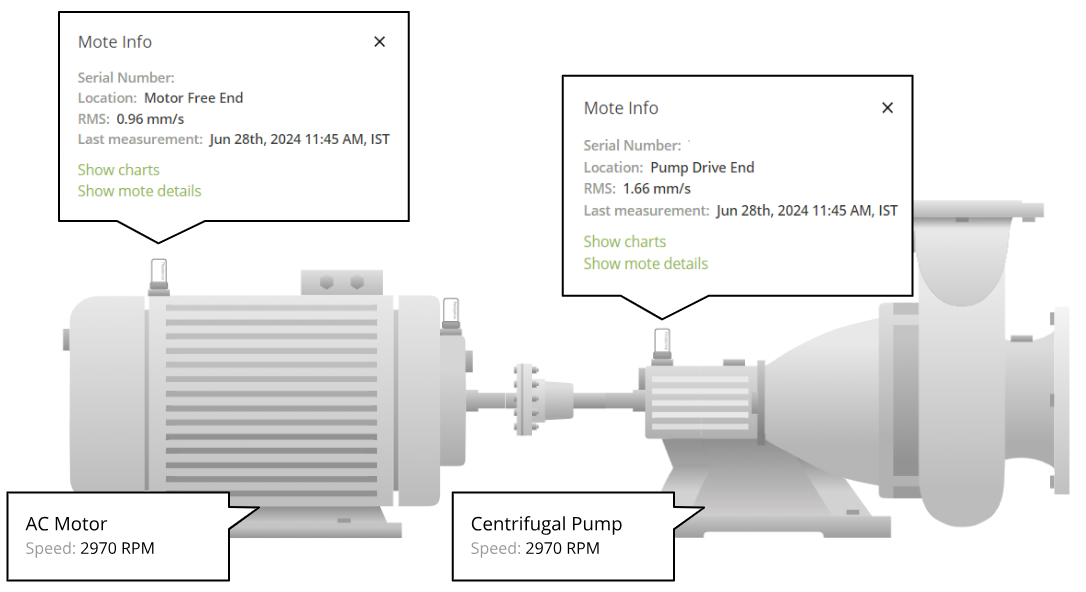
With continuous vibration monitoring, the Petasense system detected early signs of bearing distress at the motor drive-end. An automated alert was sent to the site team based on elevated vibration levels. Further analysis using the demodulated spectrum identified clear bearing defect frequencies, specifically at the Ball Pass Frequency Inner Race (BPFI), indicating an inner race fault developing in the motor bearing.
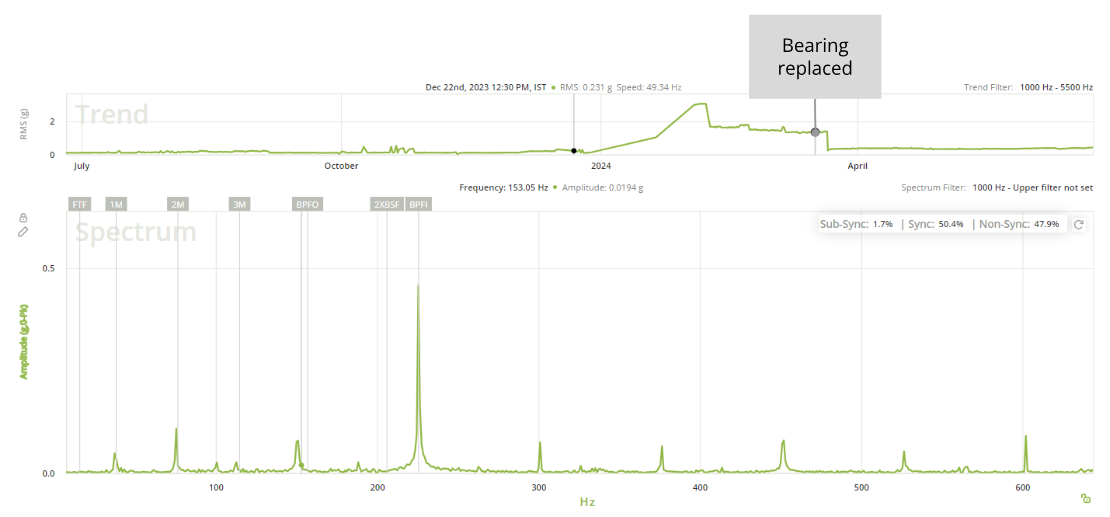
After confirming the diagnosis, the maintenance team proactively replaced the faulty bearing. Post-replacement monitoring confirmed a marked reduction in vibration levels, validating that the intervention was successful.
This case highlights how IIoT-enabled condition monitoring enabled early fault detection, timely maintenance, and uninterrupted production of critical medications, demonstrating the value of predictive maintenance in pharmaceutical operations.
Case Study 2: Agitator – Looseness and Misalignment
Agitators are essential in pharmaceutical manufacturing, where they ensure uniform mixing, dissolution, and homogenization of ingredients. Their role is critical in maintaining batch consistency and product quality across formulations.
One such agitator used for blending critical ingredients began to exhibit abnormal vibration patterns. The Petasense system detected elevated RMS velocity levels, exceeding 20 mm/s (0.78 in/s), along with a strong 1x vibration peak in a single direction. Analysis pointed to mechanical looseness at the base and possible coupling misalignment. If unaddressed, these conditions could have led to inconsistent mixing, reduced product quality, and long-term equipment damage.
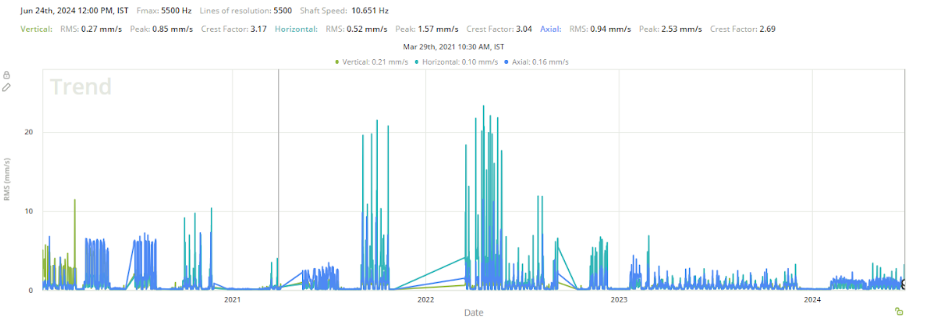
The site maintenance team responded by tightening the agitator base and realigning the shafts. Follow-up vibration data confirmed a substantial drop in vibration levels, validating the success of the repair. This proactive intervention ensured reliable agitator performance, safeguarded batch quality, and prevented unplanned production downtime. The case highlights the importance of continuous condition monitoring and the ability to act on early warning signs before they escalate into failures.
Case Study 3: Vacuum Pump – Overheating
Vacuum pumps play a crucial role in the lyophilization (freeze-drying) process, which is essential for preserving the stability and efficacy of sensitive biological and pharmaceutical products. During this process, vacuum pumps create and maintain a low-pressure environment within the freeze-dryer chamber, enabling the removal of moisture without compromising the integrity of the final product.
At a large pharmaceutical manufacturing site, one of several vacuum pumps used in lyophilization triggered a temperature alarm in Petasense ARO. Follow-up trend analysis revealed a sustained rise in temperature at both the gearbox and pump housing. Since overheating in this equipment could disrupt the low-pressure environment required for freeze-drying, this posed a significant risk to production efficiency and product quality.
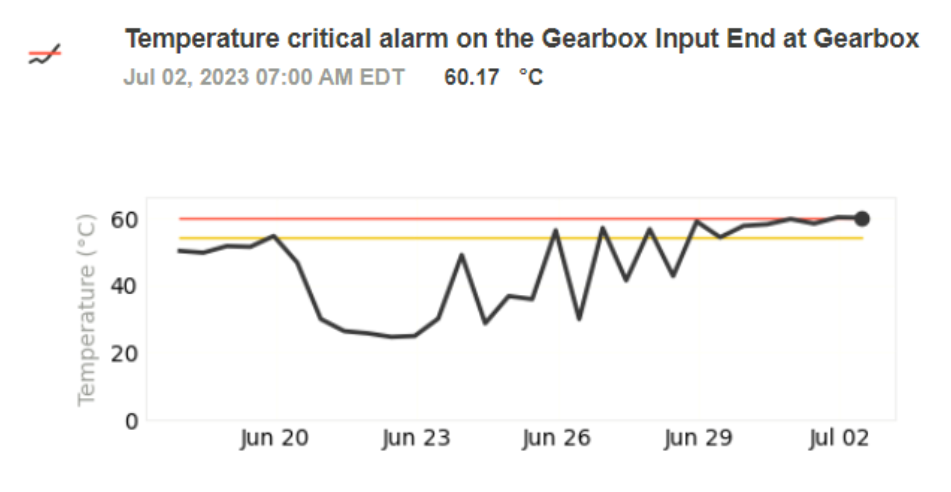
With real-time visibility from the Petasense platform, the plant’s maintenance team quickly investigated the anomaly. They considered potential causes such as increased loading, flow irregularities, and other process-related changes. To rule out internal wear, the team also performed oil analysis to check for elevated levels of wear metals.
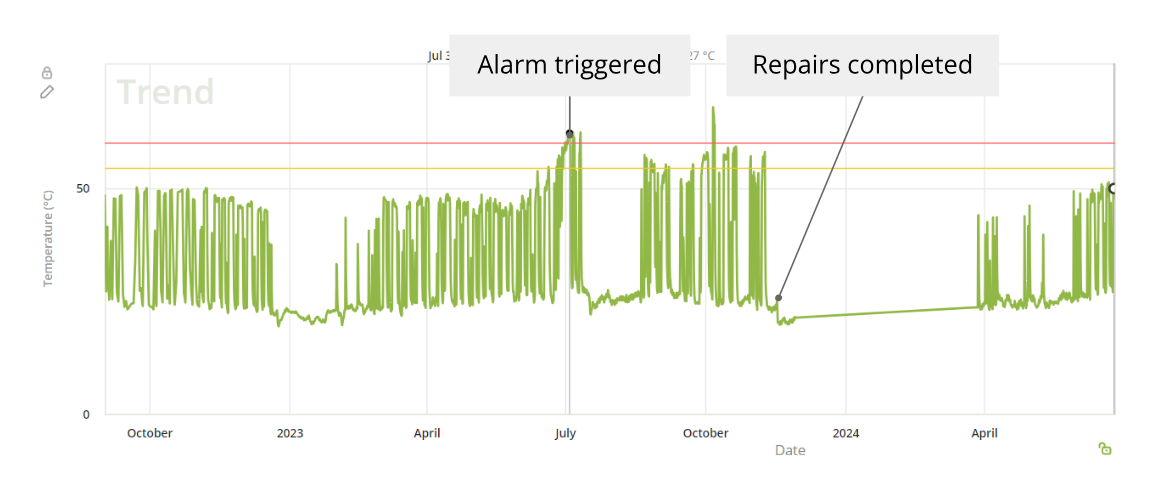
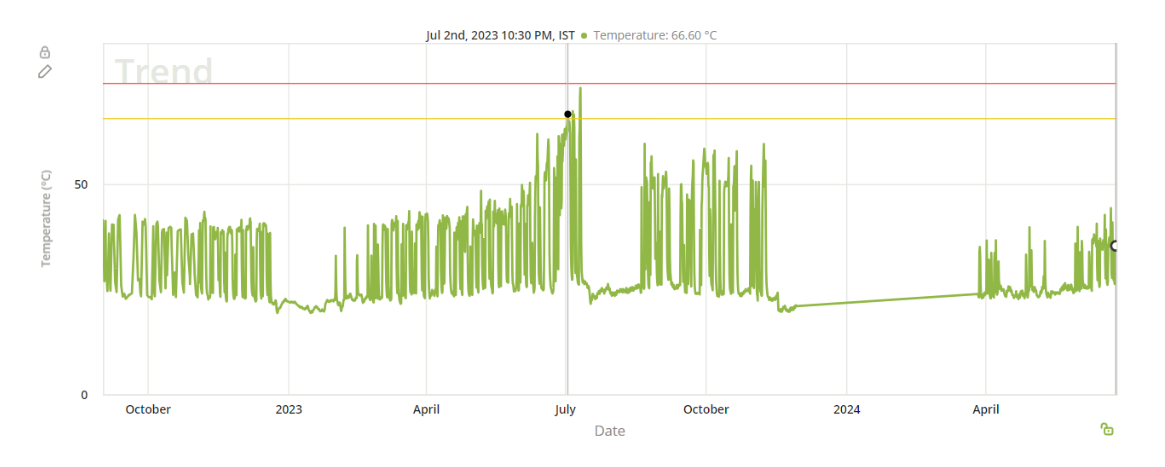
Ultimately, the decision was made to proactively replace the skid. Post-replacement monitoring showed a return to stable operating temperatures, confirming the effectiveness of the intervention and preventing any impact on product output. This case highlights how continuous monitoring and timely action can help preserve equipment reliability and maintain high production standards in pharmaceutical environments.
Key Takeaways
Predictive Maintenance is no longer a future ambition—it’s actively helping pharmaceutical manufacturers reduce downtime, ensure GMP compliance, and maintain reliable operations across production and utility assets.
With centralized monitoring, intelligent analytics, and pharma-specific features, platforms like Petasense ARO are enabling maintenance teams to make smarter, faster decisions at scale.
From detecting bearing faults in glycol pumps to resolving alignment issues in agitators and overheating in vacuum pumps, the case studies we’ve shared demonstrate how real-time insights are driving real-world results in pharmaceutical environments.


 Thanks for subscribing - stay tuned for our next newsletter
Thanks for subscribing - stay tuned for our next newsletter